There are several methods to determine Aerodynamic Particle Size Distribution (APSD), such as laser diffraction and time of flight, but the most common industry-accepted practice is using Cascade Impactors.
Andersen Cascade Impactor with Pre-separator for Inhalation Powders and Nasal Powders (DPIs)
General information:
Dry Powder Inhalers (DPIs) are another type of inhalation drug delivery system, similar to Metered Dose Inhalers (MDIs). However, there are distinct differences in their operation and challenges in their testing:
- Flow Resistance. DPIs offer varying degrees of resistance to flow. This means that different inhalers will require different levels of patient effort for inhalation. The inherent resistance in each DPI design stems from factors like device geometry, mouthpiece design, and powder properties. Therefore, the testing setup for DPIs should accommodate this variable resistance to mimic patient usage accurately.
- Volume Setting. The integration of adjustable flow timings based on specific volumes, such as 2 or 4 litres, is crucial. This is because it helps simulate the inhalation volume of a typical patient. By setting the volume, testers can ensure the drug delivery during testing closely mimics the real-world usage of the DPI by patients.
- Working airflow. For DPIs, the air flow rate is much higher than for MDIs (28,3; 60; 90 L/min, ecc).
- Sampling & Testing. Unless a specific monograph indicates otherwise, it’s standard to test the drug content from 10 separate units. This ensures consistency across multiple units of the same product. From each of these units, drug content determinations are made at two distinct stages: at the beginning of the unit’s lifespan and at the end. This ensures that the DPI provides a consistent dose throughout its entire life. As a result, from 10 separate units, a total of 20 determinations will be made (2 from each).
By conducting such rigorous testing on DPIs, manufacturers and regulatory bodies can ensure the consistency and efficacy of the drug delivery to the patient. DPIs’ unique challenges, like varying flow resistance, necessitate specialized testing protocols to ensure they meet the therapeutic needs they are designed for.
System:
For testing of DPIs Andersen Cascade Impactor or Next Generation Impactor with Pre-separator are used. Flowrate is 28,3; 60 or 90 L/min depending on the inhaler’s type (most commonly 60 or 90 L/min) for ACI 8 Stage and 30; 60 or 100 (most commonly 60 or 100 L/min) for Next Generation Impactor.
Cut-Off Diameters (μm) for Andersen Cascade Impactor with and without Pre-Separator at 28.3 L/min Compared with Use at 60 and 90 L/min:
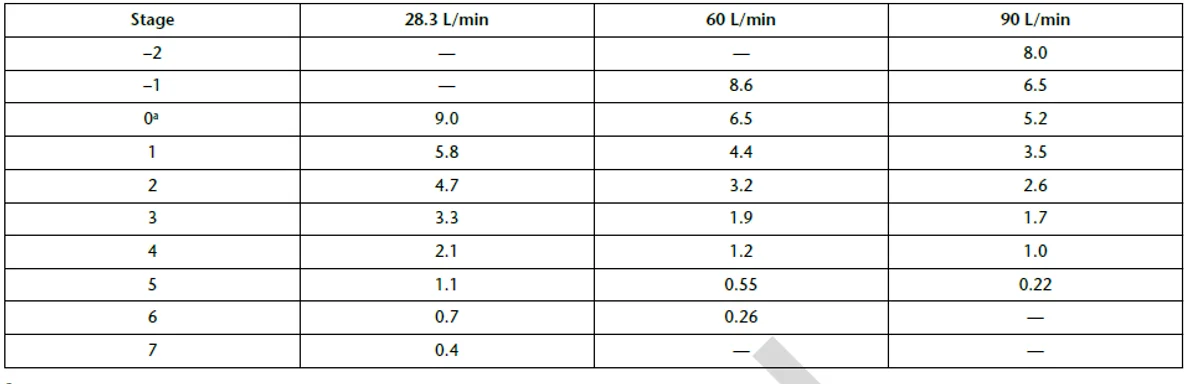
Cut-off diameters (μm) for Next Generation Impactor with and without Pre-separator at 30, 60, and 100 L/min:
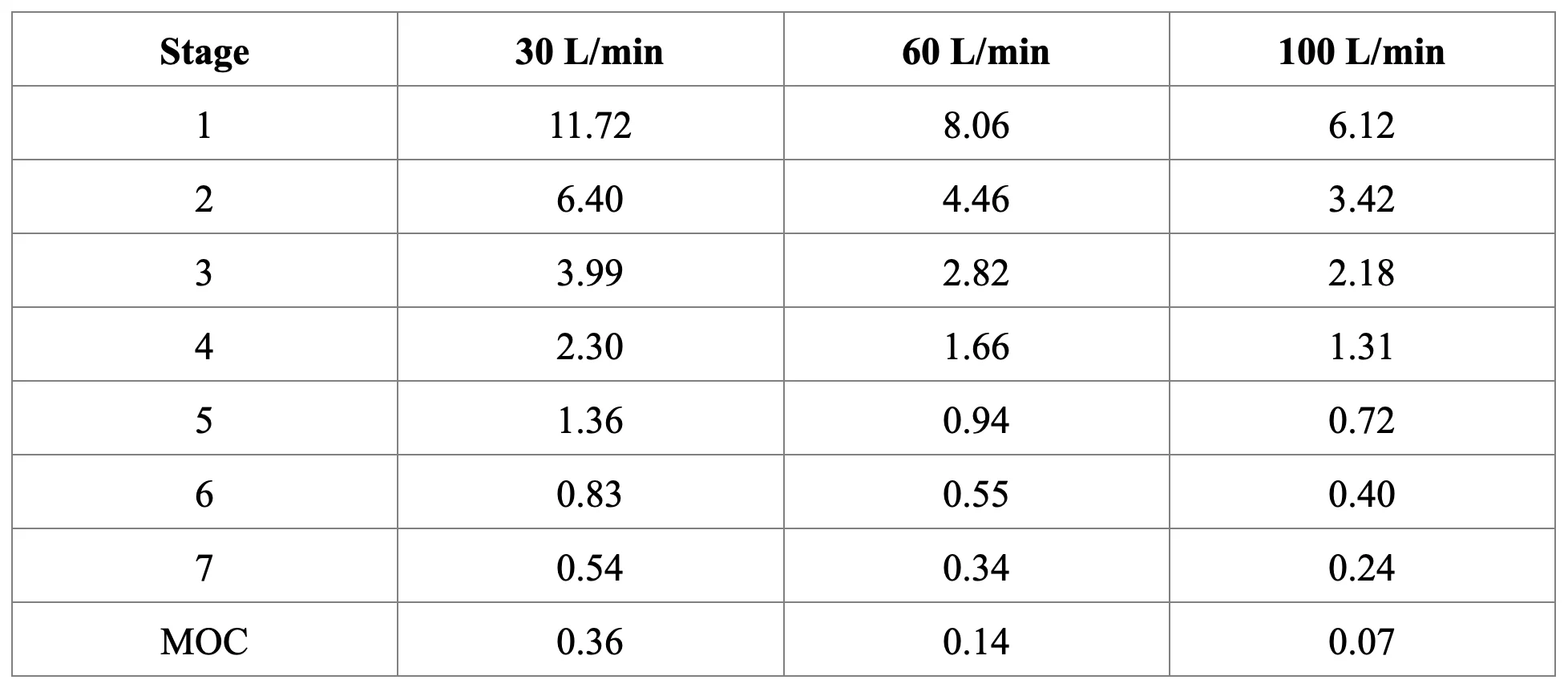
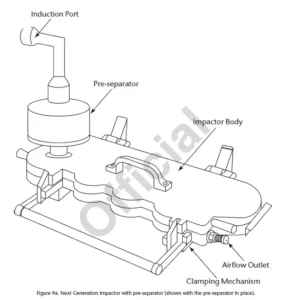
Procedure:
- Integrate the cascade impactor with the specified control system.
- Ensure effective particle capture by appropriately coating the particle collection surface of each stage with glycerol, silicone oil, or a similar liquid.
- Construct the impactor according to the manufacturer’s instructions, incorporating an after-filter below the final stage to capture any fine particles that may otherwise escape.
- Introduce a predetermined volume (up to 10 mL) of a suitable solvent into the pre-separator, or apply a coating to the particle collection surfaces of the pre-separator to prevent re-entrainment of impacted particles.
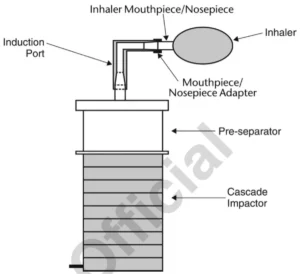
- Affix a mouthpiece/nosepiece adapter to the induction port’s end to create an airtight seal between the product’s mouthpiece/nosepiece and the induction port.
- To calibrate the flow rate, activate the vacuum pump and open the two-way solenoid valve. Measure the flow rate through the system
- Attach a flowmeter to the induction port. Ensure the flowmeter is calibrated for volumetric flow.
- Adjust the flow-control valve until a consistent flow through the system is achieved at the specified rate.
- Prepare the inhalation powder or nasal powder according to the instructions provided on the label.
- With the vacuum pump in operation and the two-way solenoid valve closed, insert the product’s mouthpiece/nosepiece, held horizontally, into the mouthpiece/nosepiece adapter.
- With the product in position and the intended flow activated, measure the absolute pressure on both sides of the flow-control valve.
- Once the product is properly placed, release the powder into the apparatus by opening the two-way solenoid valve for the required duration, sampling 4.0 L of air based on the determined duration in seconds for testing Delivered Dose Uniformity.
- After sampling a total volume of 4.0 L of air per actuation, close the two-way solenoid valve and remove the product.
- Upon completion of the final actuation, detach the product from the mouthpiece/nosepiece adapter and deactivate the vacuum pump.
- Disassemble the apparatus meticulously. Rinse the drug residue from the mouthpiece/ nosepiece adapter, induction port, pre-separator, each stage, and the collection plate of each stage, as well as the filter, directly into appropriately sized flasks, using a suitable solvent.
- Dilute each flask quantitatively to an appropriate volume.
- Utilize a validated method of analysis to determine the mass of drug collected in each sample.

The introduction of an integrated system by InPharmaTEC streamlines the testing process for inhalation products, such as MDIs and DPIs. By combining critical components—flowmeter, solenoid valve, timer, vacuum pump, differential pressure transducer, critical flow controller (for both, MDIs and DPIs), into one unit, the workflow is indeed simplified, reducing potential sources of error and enhancing efficiency.
Benefits:
- Efficiency. By merging multiple components into a single unit, the setup and calibration time is likely reduced. This speeds up the entire testing process.
- Accuracy. A single integrated unit reduces the potential for errors that might arise when connecting multiple separate devices.
- Consistency. With an integrated system, every test is performed under identical conditions, ensuring consistent results.
- Ease of Use. Operators only need to become familiar with one integrated device rather than multiple separate components, making training and daily use simpler.
Space Saving. Combining multiple devices into one means a more compact footprint in the laboratory.
At TCR Tecora, InPharmaTEC, our dedication is unwavering towards our customers and patients with respiratory diseases. We recognize the unique challenges they face, and we’ve committed ourselves to deliver innovative and compassionate solutions that enhance their quality of life. We have meticulously streamlined our processes to ensure that every moment counts, delivering efficient healthcare solutions that prioritize the well-being of those we serve.
Click to Download the datasheet





