There are several methods to determine Aerodynamic Particle Size Distribution (APSD), such as laser diffraction and time of flight, but the most common industry-accepted practice is using Cascade Impactors.
Andersen Cascade Impactor without Pre-separator for Inhalation Aerosols, Inhalation Sprays and Nasal Aerosols. MDIs
General information:
Metered Dose Inhalers (MDIs) are commonly used delivery devices for medications intended for respiratory conditions such as Asthma and Chronic Obstructive Pulmonary Disease (COPD).
It is critical to ensure that the drug is delivered effectively and consistently to achieve the desired therapeutic effects.
For suspension or solution nasal aerosols and nasal sprays, it’s essential to analyze the droplet/particle size distribution of the emitted plume following delivery under specific experimental conditions. If the density of the liquid remains constant regardless of droplet size, volume-based size distributions can be considered equivalent to mass-based size distributions for data interpretation purposes.
System:
For testing of MDIs Andersen Cascade Impactor or Next Generation Impactor without Pre-separator are used. Flowrate is 28,3 L/min.
Cut-Off Diameters (μm) for Andersen Cascade Impactor with and without Pre-Separator at 28.3 L/min Compared with Use at 60 and 90 L/min:
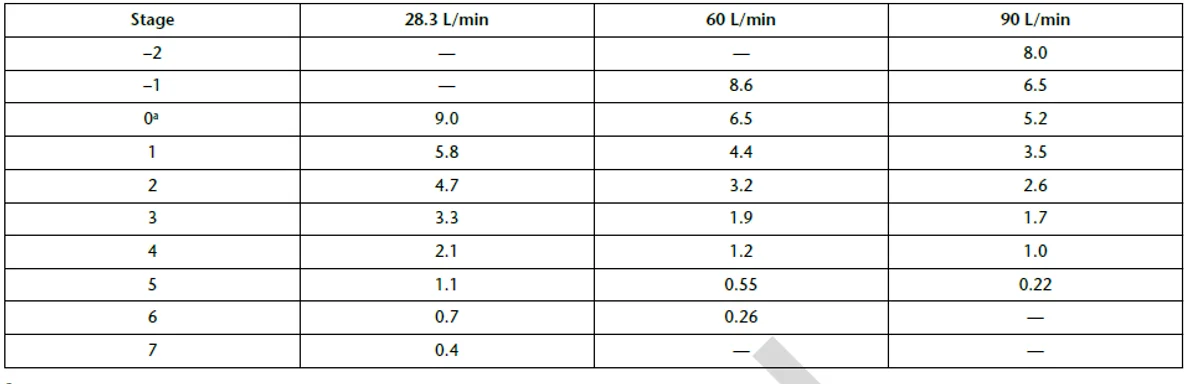
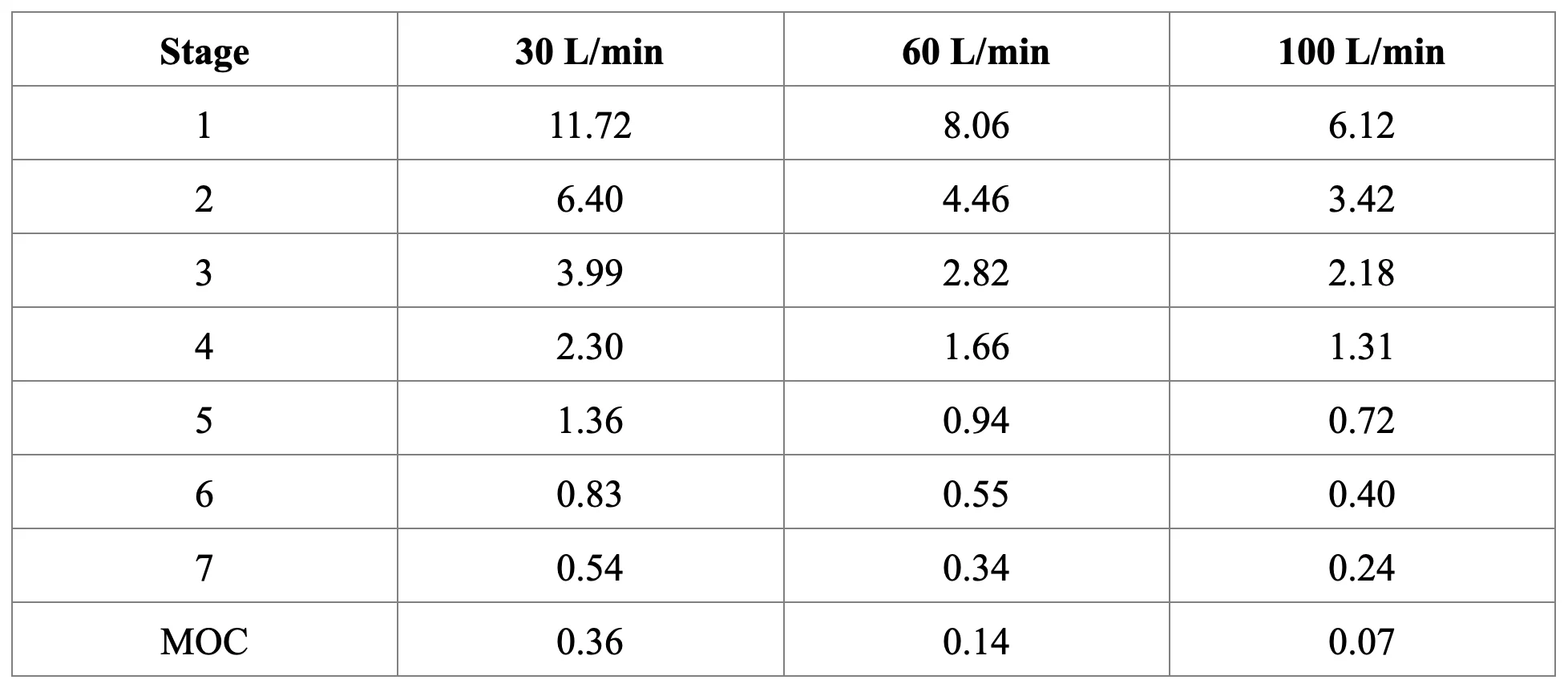
Cut-off diameters (μm) for Next Generation Impactor with and without Pre-separator at 30, 60, and 100 L/min:
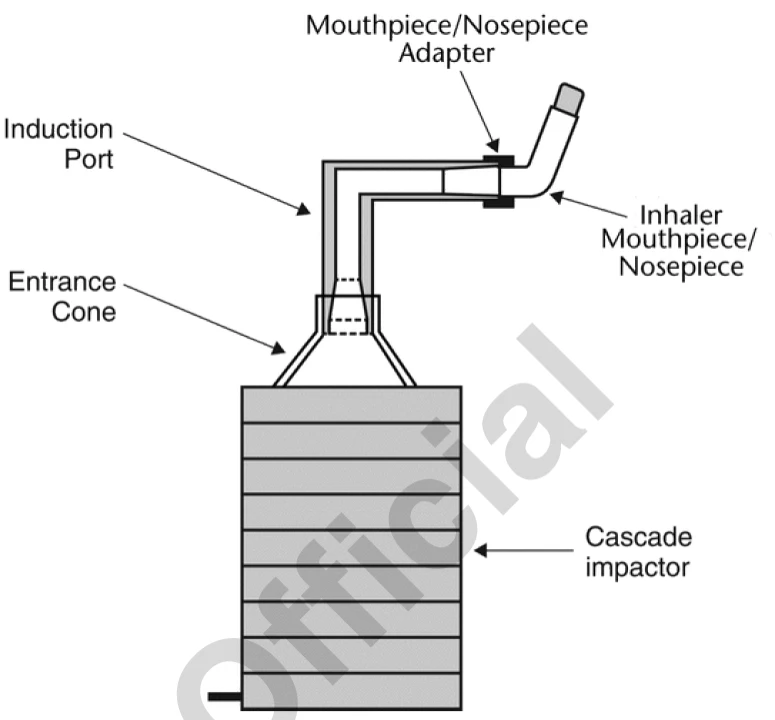
Procedure:
- Set up the multistage cascade impactor.
- Ensure efficient particle capture by coating the particle collection surface of each stage with glycerol, silicone oil, or another suitable liquid.
- Connect the mouthpiece/nosepiece adapter securely to the induction port to create a tight seal between the product mouthpiece/nosepiece and the induction port.
- Guarantee tight seals between the different stages of the cascade impactor to prevent any leakage.
- Activate the vacuum pump to draw air through the cascade impactor and calibrate the flow rate through the system using an appropriate flowmeter attached to the open end of the induction port.
- Adjust the flow-control valve on the vacuum pump to maintain a consistent flow through the system at 28.3 L/min (±5%).
- Prepare the product for use according to the label instructions for shaking, priming, and firing.
- While the vacuum pump is operational, insert the mouthpiece/nosepiece of the inhaler into the mouthpiece/nosepiece adapter and promptly administer the first of the minimum. recommended number of actuations into the cascade impactor.
- Remove the product from the mouthpiece/nosepiece adapter.
- Cleanse the mouthpiece/nosepiece adapter and the induction port using an appropriate solvent, and dilute quantitatively to an appropriate volume.
- Disassemble the cascade impactor, place each collection plate or filter in a separate container, and rinse the drug residue from each component.
- Dilute each sample quantitatively to an appropriate volume. Utilize a validated method of analysis to ascertain the mass of drug collected in each component.

The introduction of an integrated system by InPharmaTEC streamlines the testing process for inhalation products, such as MDIs and DPIs. By combining critical components—flowmeter, solenoid valve, timer, vacuum pump, differential pressure transducer, critical flow controller (for both, MDIs and DPIs), into one unit, the workflow is indeed simplified, reducing potential sources of error and enhancing efficiency.
Benefits:
- Efficiency. By merging multiple components into a single unit, the setup and calibration time is likely reduced. This speeds up the entire testing process.
- Accuracy. A single integrated unit reduces the potential for errors that might arise when connecting multiple separate devices.
- Consistency. With an integrated system, every test is performed under identical conditions, ensuring consistent results.
- Ease of Use. Operators only need to become familiar with one integrated device rather than multiple separate components, making training and daily use simpler. Space Saving. Combining multiple devices into one means a more compact footprint in the laboratory.
At TCR Tecora, InPharmaTEC, our dedication is unwavering towards our customers and patients with respiratory diseases. We recognize the unique challenges they face, and we’ve committed ourselves to deliver innovative and compassionate solutions that enhance their quality of life. We have meticulously streamlined our processes to ensure that every moment counts, delivering efficient healthcare solutions that prioritize the well-being of those we serve.
Click to Download the datasheet




